Experimental and theoretical investigation of the treatment of Cu-rich acid mine drainage using iron oxide magnetic nanoparticles
Resumen
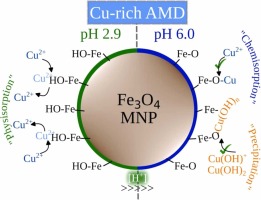
Acid Mine Drainage (AMD) is a significant environmental problem in the mining industry due to its high concentration of hazardous metals and metalloids, sulfate compounds, and low pH levels. Despite the attention that iron oxide magnetic nanoparticles (MNP) have received for AMD remediation, there is still a lack of understanding of the physicochemical mechanisms behind their non-specific adsorption, particularly in distinct variations of AMD, such as Cu-rich AMD. In this study, we synthesized, characterized, and applied MNP to the two-step treatment of Cu-rich AMD. The chemical and physical properties of the MNP and magnetically separated sludges after AMD treatment are characterized. Additionally, the chemical species adsorbed onto the MNP, the oxidation state of the resultant sludge after Cu-rich AMD treatment, and the short-range ordering of metal contaminant species on the surface of the MNP are identified. Finally, first-principles calculations using Density Functional Theory were conducted to understand how different Cu ion species adsorb to the MNP surface depending on the pH of the Cu-rich AMD. The bonding between MNP and Cu species occurs primarily through metal cation-oxygen bonds on the surface of MNP, and this bonding is influenced by the pH of the solution. A combination of experimental and theoretical approaches was the key to arrive at this conclusion. This information can aid in the comprehension of how metal contaminants adhere to the surfaces of MNP and in the precise engineering of these nanoparticles.
Full citation
N. Naveas, R. Pulido, V. Torres-Costa, F. Agulló-Rueda, M. Santibáñez, F. Malano, G. Recio-Sánchez, K. A. Garrido-Miranda, M. Manso-Silván and J. Hernández-Montelongo,
“Experimental and theoretical investigation of the treatment of {Cu}-rich acid mine drainage using iron oxide magnetic nanoparticles,”
J. Environ. Chem. Eng. 12, 113822 (2024).
DOI: 10.1016/j.jece.2024.113822